Paragard IUDs Under Legal Scrutiny: Is It Time to Look for Safer Alternatives?
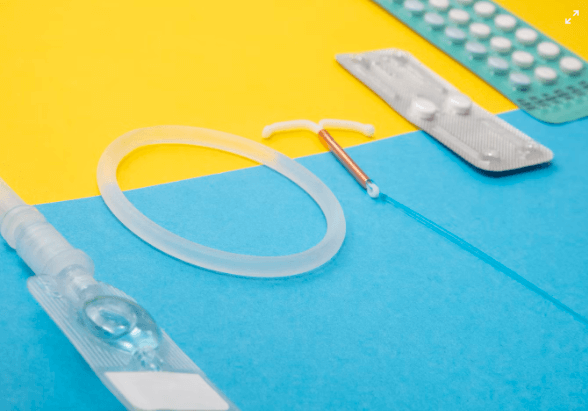
Long-acting reversible contraceptives (LARCs) like intrauterine devices (IUDs) are highly effective forms of birth control. However, some models have faced safety concerns that have led to legal scrutiny and calls for reform.
The Paragard IUD, one of the oldest copper IUDs on the market, has faced thousands of injury lawsuits alleging harmful side effects. As medical technology advances and women demand options that put their well-being first, is it time for regulators and clinicians to critically re-examine widely used devices like Paragard?
This article explores the legal challenges facing Paragard. We will also look at emerging safer alternatives that could reshape the future of long-acting birth control.
How Does the Copper IUD Work?
The copper IUD is a small, T-shaped device placed in the uterus by a healthcare provider to prevent pregnancy for up to 10 years. It works by releasing copper into the uterus, which changes the cervical mucus and uterine lining to create an environment that is uncomfortable for sperm.
The copper IUD works primarily by preventing fertilization from occurring. It releases copper ions that alter the environment inside the uterus, making it very difficult for sperm to survive or reach an egg. Studies have found the copper IUD to be over 99% effective at stopping pregnancy.
IUDs come in two primary kinds: those which solely contain copper and those which contain hormones. The copper IUD works entirely on copper and does not produce any hormones. When inserted into the uterus, the copper ions deter sperm and make the natural environment inhospitable for fertilization and implantation.
A medical provider must insert and remove the copper IUD during an in-office procedure that typically only takes a few minutes.
Once placed, the IUD can remain highly effective for up to 10 years if the woman wants long-term contraceptive protection without needing to think about it daily. She has the option of having it taken out at any time if she wants to attempt pregnancy or switch methods.
If a copper IUD is implanted within five days of unprotected sex, it can be used as emergency contraception in situations of crisis to avoid possible pregnancy. When placed promptly after intercourse, it greatly reduces the likelihood of an accidental pregnancy occurring.
The most common copper IUD available in the United States is called ParaGard. Other countries offer additional copper IUD brands as well. Once inserted, it provides highly effective birth control for many years without hormones or user action.
Why People Are Filing Lawsuits Against Paragard IUD?
According to TorHoerman Law, while IUDs can be a safe long-term birth control option, some women have experienced serious injuries from the Paragard IUD manufactured by Teva Pharmaceuticals.
Complaints include internal bleeding, infections, and organ damage resulting from the Paragard fracturing or breaking during insertion or removal.
These complications sometimes require additional medical treatment and can lead to ongoing health issues. Women who have been injured by a defective Paragard IUD may file a personal injury lawsuit against the manufacturer.
To recover money for medical expenses, missed income, pain, and suffering, and other harms brought on by the device would be the aim of a Paragard lawsuit. Attorneys estimate Paragard IUD lawsuit settlement amounts could range from $10,000 to $400,000 based on prior case outcomes, though each case is unique.
Damages claimed in a Paragard lawsuit may cover past and future medical bills, loss of income, permanent disabilities, and non-economic damages like reduced quality of life. The lawsuit could also pursue punitive damages if plaintiffs can show the company failed to address known safety issues.
While IUDs are normally a very safe contraceptive, these lawsuits aim to hold Teva Pharmaceuticals accountable for any women injured by a defective Paragard IUD product. Compensation through a potential settlement could help cover costs related to the injuries suffered.
Read also A Journey through Cape Town’s Premier Rehab Centres
Non-Hormonal Birth Control: A Safer Alternative
Cervical Cap
The cervical cap is a barrier method of contraception that sits over the cervix. It is dome-shaped and made of silicone. Like the diaphragm, a healthcare provider must fit each cap individually. The cap should be used in conjunction with spermicide.
Effectiveness rates estimate that about 20 out of every 100 women using the cervical cap as their sole method of birth control will become pregnant within a year.
One benefit is that the cap can remain in place for up to two days after intercourse. This allows for spontaneous intimacy. Fertility is not delayed, so trying to conceive can occur at any time by simply removing the cap.
Spermicide
Spermicide is a chemical contraceptive option available over the counter. Upon contact, it is intended to render sperm immobile or fatal. Gels, foams, and suppositories that are placed into the vagina before sexual activity are examples of spermicide forms.
If used alone as the sole method of birth control, statistics estimate spermicide fails in preventing pregnancy around 28% of the time. However, it can boost the effectiveness of other contraceptives like condoms, diaphragms, and cervical caps when used together.
One potential drawback is an allergic or sensitive reaction to nonoxynol-9, the main active ingredient in most spermicides.
Leaving the spermicide in place for at least 8 hours after intercourse is recommended, though some leakage may occur. Importantly, it does not provide protection against sexually transmitted diseases. There is even a risk of infections becoming more likely if the spermicide irritates the vaginal lining.
Sponge
The contraceptive sponge is a soft foam disk containing spermicide. It is designed to be inserted into the vagina prior to intercourse. The sponge has a nylon loop attached for easy removal afterward. Most pharmacies sell sponges without a prescription over the counter.
By obstructing the cervix, the sponge prevents sperm from entering the uterus and thereby prevents pregnancy. It also releases spermicide that immobilizes sperm on contact.
Statistics from sexual health organizations indicate the sponge is less effective for those with a prior pregnancy history. For individuals with no previous live births, the failure rate is about 9% with perfect use and 14% with typical use.
In contrast, for those previously pregnant, the failure risk increases to approximately 20% with ideal use and 22% in regular use cases.
Potential risks include a higher chance of yeast infections as well as toxic shock syndrome if left in place beyond 30 total hours. Common side effects can also be vaginal dryness or allergic reactions to sponge materials.
FAQs
1. Why don’t doctors recommend the copper IUD?
A: Doctors typically advise against using the non-hormonal copper IUD for individuals who have or suspect they have an STI or pelvic infection. They also recommend against its use for those who have experienced a pelvic infection within the last 3 months or have untreated cervical or uterine cancer.
2. Why is Mirena considered better than copper IUDs?
A: Mirena, containing levonorgestrel, operates differently from the copper IUD (Paragard). While Paragard utilizes copper to create an inhospitable environment for sperm and egg fertilization, Mirena thickens cervical mucus, slows sperm movement, and reduces the thickness of the endometrial lining.
3. How painful is the insertion of Paragard?
A: Pain levels vary among individuals. Some women may experience discomfort during or after insertion. As Paragard is inserted, you may feel cramping or pinching, and some women might experience feelings of faintness, nausea, or dizziness for a few minutes afterward.
While ongoing innovation will hopefully lead to safer birth control options, it is crucial not to lose sight of the fact that contraception access remains essential for empowering women. It helps in promoting women’s autonomy and equality.
By addressing safety issues transparently and prioritizing clinical feedback, manufacturers can help ensure methods like IUDs fulfill their potential to improve lives substantially. This approach also gives all users confidence in their health and well-being.